Nickel plating can add durability, hardness, conductivity, and heat-resistance to your product, in addition to protecting against corrosion and enhancing its aesthetic appeal. But before you enlist plating services from a metal finisher, you must first decide between two plating methods: electrolytic or electroless plating.
The following guide explains both processes, how they differ, and their distinct advantages.
What Are Electrolytic and Electroless Nickel Plating?
Traditional electrolytic nickel plating requires a catalyst and a direct current (DC) charge to start a chemical chain reaction to coat an object (the substrate) with a thin layer of nickel—however, with electroless nickel plating, no catalyst or charge is needed. Instead, electroless formulas include a chemical reducing agent (phosphorous) that allows the user to coat the substrate without further processing.
Both methods add a thin layer of nickel to the target surface, but electroless nickel plating (ENP) provides additional wear- and corrosion-resistance, lubricity, and ancillary performance characteristics compared to its electrolytic counterpart. Also known as autocatalytic coating, ENP can be used on projects with tight tolerance specifications and is easy to apply in uniform layers.
Electrolytic nickel plating, on the other hand, is typically thicker around the substrate corners and edges and can’t deliver the same level of precision. During electrolytic plating, deposit density is controlled by the length of time the product is submerged and how many amps per square foot are applied.
Process of Nickel Plating
First, the substrate material must be cleaned and pretreated before it can be plated with nickel. Pretreatments vary depending on the substrate type and the product’s intended use.
Next, the product is placed into a plating bath, which consists of positively charged, dissolved nickel phosphorus. The substrate automatically draws the positively charged nickel ions onto its surface, completing a fine layer of coating. Electroless nickel plating does not require electricity, nor does it need constant filtration to keep debris from adhering to the surface.
Industries That Use Electroless Nickel Plating
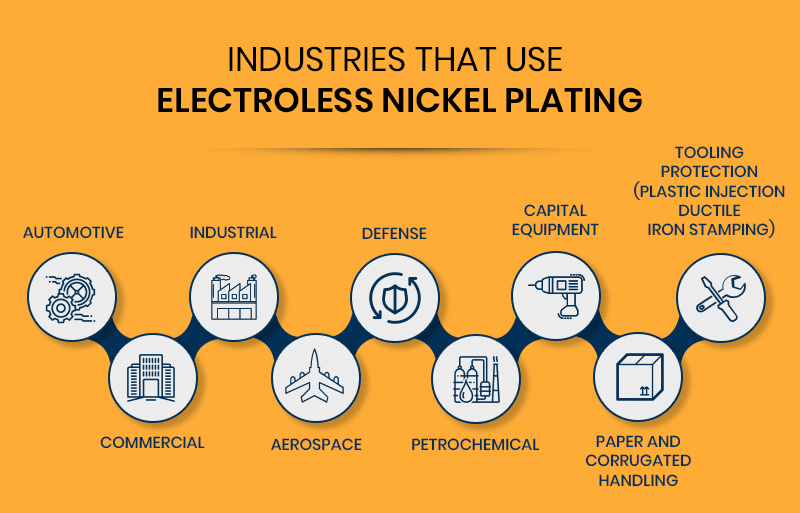
Electroless nickel plating is common among these industries:
- Automotive
- Commercial
- Industrial
- Aerospace
- Defense
- Petrochemical
- Capital equipment
- Paper and corrugated handling
- Tooling protection (plastic injection ductile iron stamping)
Advantages of Electroless Nickel Plating
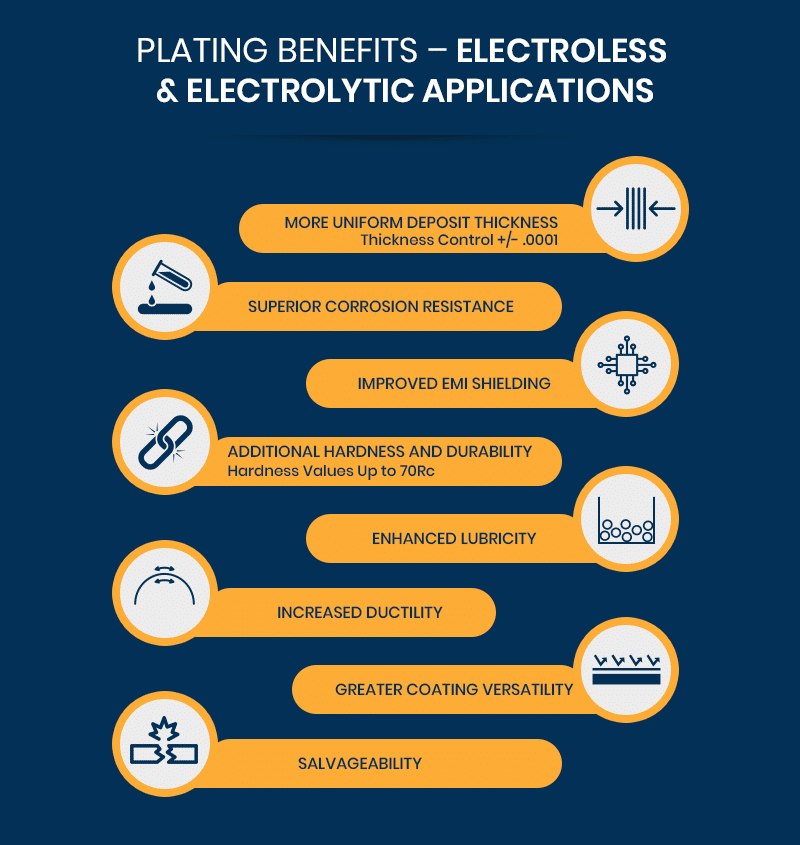
Electroless nickel plating offers numerous advantages over traditional electrolytic nickel plating, including:
- More Uniform Deposit Thickness: Electroless nickel plating is more precise than electrolytic plating with tolerances of +/- .0001 inches. It can be used to create complex geometries and avoids a common problem called the “dog bone effect,” which occurs when too many amps per square foot are applied during electrolytic plating, resulting in an inconsistent deposit.
- Superior Corrosion Resistance: ENP offers better corrosion-resistance thanks to the introduction of phosphorus to the solution.
- Improved EMI Shielding: Phosphorus also provides magnetic properties that allow metal finishers to control how much electromagnetic interference occurs around the substrate. This feature has proven vital for applications that involve electronics.
- Additional Hardness and Durability: ENP deposits can be treated with heat to reach approximately 90% of the same hardness as chromium. Low-phosphorus ENP coatings measure up to 63 on the Rockwell scale (Rc) as-plated. By comparison, type II bright nickel deposits created with electrolytic plating have an as-plated hardness of 50+ Rc.
- Enhanced Lubricity: Electroless nickel plating also creates less friction against other materials, resulting in better lubricity and reduced surface scarring.
- Increased Ductility: Because ENP is more ductile than traditional nickel coating, it’s less likely to crack, break, or shatter under stress. Type I pure nickel plating, however, provides comparable ductility and can meet or exceed AMS2424 specifications established by SAE International.
- Greater Coating Versatility: Electroless nickel plating can be applied to virtually all metallic substrates, and there aren’t any limits to how thick the layer can be.
- Salvageability: ENP is also an excellent choice for materials that will be salvaged later.
Electrolytic Plating Process
Just like with electroless nickel plating, electrolytic plating also begins with cleaning and pretreating the substrate. Next, the product is placed into a bath with a conductive base and positively charged nickel. Once the object is submerged, an external electrical current or rectifier is administered to the solution. Electricity charges the nickel anodes, causing them to release ions that attach to the surface of the substrate, thereby completing the plating process.
Advantages to Electrolytic Plating
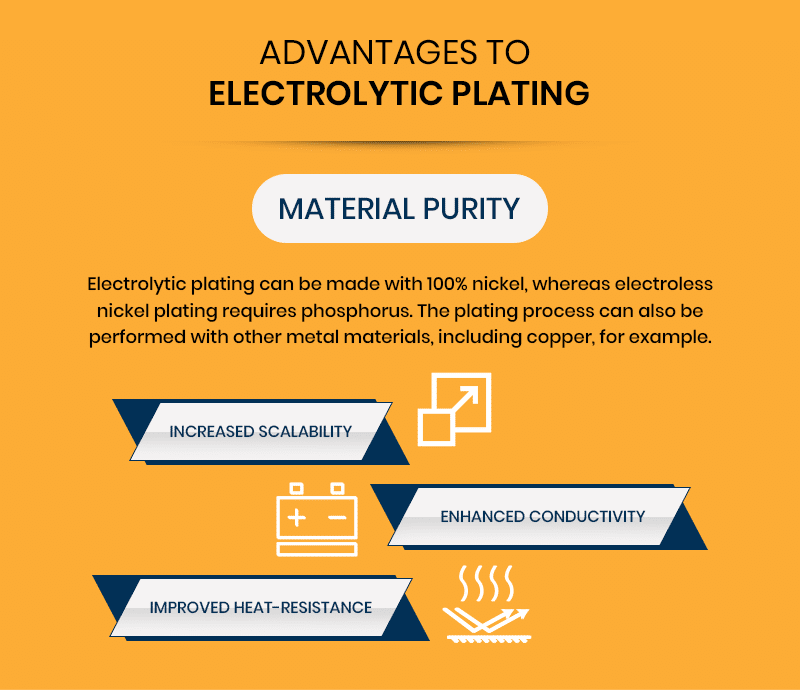
Some situations are better suited to electrolytic plating instead of electroless nickel plating. Here are a few benefits of electrolytic plating:
- Material Purity: Electrolytic plating can be made with 100% nickel, whereas electroless nickel plating requires phosphorus. The plating process can also be performed with other metal materials, including copper, for example.
- Increased Scalability: Electrolytic plating is generally less expensive than electroless nickel plating and can create higher production volumes with shorter turnaround, which makes the process slightly more productive.
- Enhanced Conductivity: A higher concentration of nickel provides better conductivity compared to electroless nickel plating.
- Improved Heat-Resistance: After processing, nickel deposits can withstand temperatures of up to 1,832°F.
How to Choose a Suitable Partner for Electroless & Electrolytic Nickel Plating
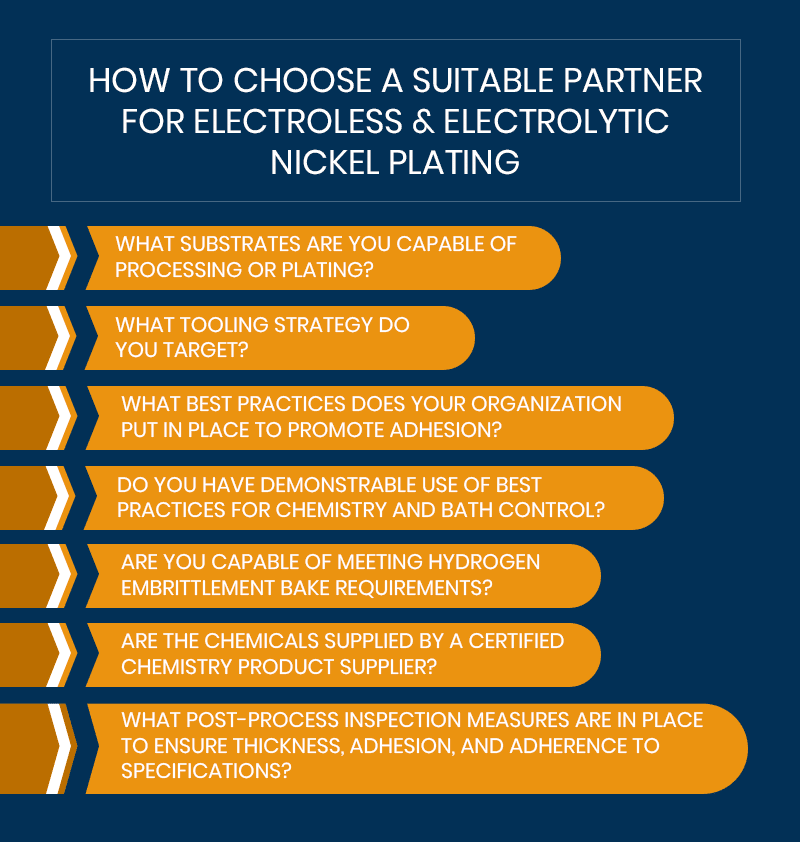
When choosing a new manufacturing partner, vet them carefully to ensure you receive the service and quality you expect. We recommend asking a prospective metal finisher the following questions:
- What substrates are you capable of processing or plating?
- What tooling strategy do you target?
- What best practices does your organization put in place to promote adhesion?
- Do you have demonstrable use of best practices for chemistry and bath control?
- Are you capable of meeting hydrogen embrittlement bake requirements?
- Are the chemicals supplied by a certified chemistry product supplier?
- What post-process inspection measures are in place to ensure thickness, adhesion, and adherence to specifications?
With over 75 years of experience, Pioneer Metal Finishing has the proven expertise to meet or exceed your metal finishing and plating needs. Find out more about our electroless nickel plating service and electrolytic nickel plating service on our website, or contact us now to discuss your specification requirements.


